One 15- to 30-minute intravenous (IV) infusion provides a full course of antiviral influenza treatment1
Use these dosing guidelines to determine the dosage of Rapivab® (peramivir injection) for your patients 6 months of age and older.
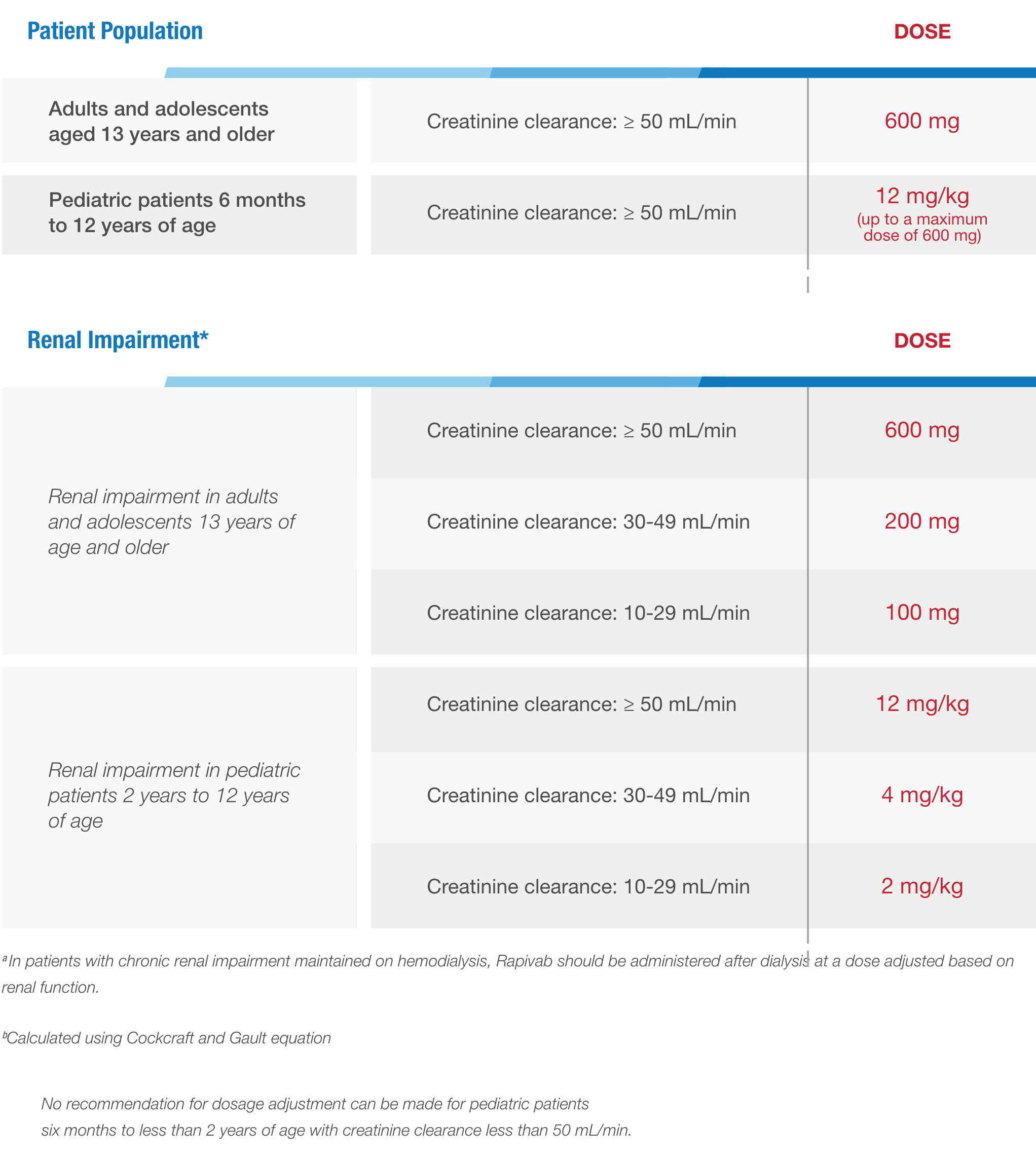
The administration of Rapivab in patients with renal impairment is at the discretion of the treating physician.
Preparation of Rapivab for IV infusion
Use aseptic technique during the preparation of Rapivab to prevent inadvertent microbial contamination. There is no preservative or bacteriostatic agent present in the solution.1
Follow the steps below to prepare a diluted solution of Rapivab1:
1. Do not use if seal over bottle opening is broken or missing.
2. Visually inspect Rapivab for particulate matter and discoloration prior to administration.
3. Dilute an appropriate dose of RAPIVAB 10 mg/mL solution [see Dosage and Administration (2.1, 2.2)] in 0.9% or 0.45% sodium chloride, 5% dextrose, or lactated Ringer’s. The maximum infusion volume is provided in Table 3. The final concentration of diluted RAPIVAB for administration should be between 1 mg/mL and 6 mg/mL.
4. Administer the diluted solution via intravenous infusion for 15 to 30 minutes.
5. Discard any unused diluted solution of Rapivab after 24 hours.
Once a diluted solution of Rapivab has been prepared, administer immediately or store under refrigerated conditions (2° to 8°C or 36° to 46°F) for up to 24 hours. If refrigerated, allow the diluted solution of Rapivab to reach room temperature, then administer immediately.
Do not use if seal over bottle opening is broken or missing.
